By – Edlyn Cardoza
11th January, 2021
The states of India, and their healthcare sector have begun gearing up for the vaccine drive of the two vaccines approved to fight against the novel Coronavirus. While states are in full swing to start the vaccine drive, Bhopal Gas Tragedy Survivors’ NGO have written a letter to the Honourable Prime Minister, as well as the Health Minister to halt the Covaxin Trials in the state.

This came in light after the death of the 45-year old who was allegedly a part of the trial.
Four organisations of the 1984 Survivors of the Bhopal Gas Tragedy, have addressed a letter to the PM and Union Health Minister, requesting them to stop the continuation of the Bharat Biotech’s coronavirus vaccine – Covaxin’s trials in the capital of Madhya Pradesh. The letter was written during the controversy of the 45-year old’s death who was a volunteer during the clinical trial of the vaccine in Bhopal. The volunteer who lost his life, was a survivor of the gas leak tragedy. The letter also stated the demand for punishment of those liable for the alleged spurning of norms, and compensation for the damages caused during the preliminary.
One of the organisations, Rashida Bee, the President of the Bhopal Gas Peedit Mahila Stationery Karmchari Sangh, told Scroll that from the 1,700 people on whom this vaccine is being tested on, at least 700 of them are people affected by the Union Carbide gas leak tragedy in Bhopal.
The letter further goes on to claim that proper guidelines were not followed, while receiving approval from the volunteers in Bhopal, most of whom were victims, and survivors of the gas tragedy. The letter also stated that in the month of December, the People’s Hospital – a private hospital, and medical college in the city had gone to the areas where several survivors resided, and announced that the vaccines were available, to enroll volunteers for the trial. The hospital also informed them that whoever would be participating in the trial, and taking the shot, would be paid a compensation of Rs.750, for the loss of daily wages, and travel expenses.
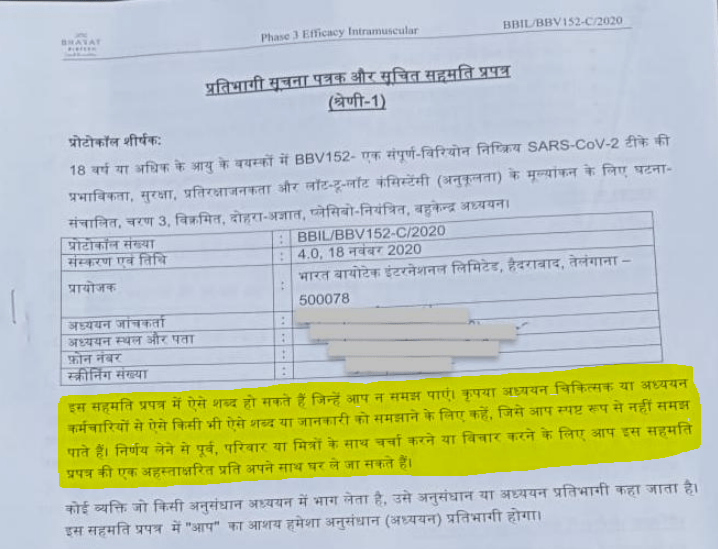
The tragedy of this issue only deepened, as the letter informed that even though it was important, the volunteers were not given copies of the consent form. Individuals were also not given the required time to read and understand, the consent form, and the information sheet. The documents of the Hindi version also contained a disclaimer where it was mentioned that, “technical language that is beyond the ability of a layperson to grasp, let alone individuals with minimal education.” The letter was sent by the four organizations of Bhopal Gas Peedit Mahila Purush Sangharsh Morcha, Children Against Dow Carbide, Bhopal Group for Information & Action, and Bhopal Gas Peedit Mahila Stationery Karmchari Sangh, who mentioned in the letter that the people who were enlisted for the trials, belonged to vulnerable groups of society, as per the guidelines of the Indian Council of Medical Research. In such cases safeguards prescribed by the ICMR, were also not taken care of.
According to the letter, the guidelines related to the participants insurance policy, and their follow-up and monitoring them after the trials, were also defied.
Bhopal Group for Information & Action’s Rachna Dhingra informed Scroll that, “ There were no records collected of the health problems, the volunteers began showcasing after the shits, and many were turned away without catering to the severe side effect the shots had on them.”
How It All Began
People in Bhopal who had volunteered for the Covaxin vaccine’s trials, alleged that they were not informed by the medical managers that they were test subjects. The volunteers said that they were persuaded that vaccines are a part of the government led vaccination drive.
People’s Medical College, recruited 1,700 members between November 28th, 2020, and December 31st, 2020. The trial was arranged in a double blind, placebo controlled trial, in which volunteers got the authentic, actual vaccine, and some got the dummy version of the shot. The participants, and the trial organisers were not supposed to be aware if a particular individual is in which of the two groups. This was done in order to calculate the efficacy level of the vaccine in preventing people from contacting COVID-19.
On Sunday, at an online press conference, Mr. Mansingh Parihar, 70 year-old, informed TheHindu that he wasn’t aware of the nature of the trial, and despite having signed the consent forms, he did not quite comprehend what was written in them. He further describes his encounter by saying that he had no illness, whatsoever, and he was fine for two days after he got the shot. But soon enough, he fell extremely sick and felt acutely breathless.
He was unsure about contacting the Principal Investigator, and the hospital, and was determinant of not going back for a second shit. He says, “The only reason I signed up was because of the Rs.750 promised and the free vaccination.”
Chandan Devi, another 60-year old volunteer, who was also a survivor of the Bhopal gas tragedy, said that she had signed up in the belief and hope that the “Corona injection” would protect her from the virus, and also because of the money they offered. She continued saying, “I started experiencing nausea, and after two days of receiving the shot, my head began spinning.”
Volunteer’s Demise
The 45-year old who had participated in the 3rd phase of the trial of Covaxin, passed away on 21st December, 2020, precisely nine days after receiving the shot. However Bharat Biotech has denied the volunteer’s death was due to the vaccine, and said, “The participant lost his life, nine days after receiving the dose, and trial reviews by the site indicate that the death is unlinked to the studying dose.” He further also went on to make a statement saying that there is no confirmation if the participant received a study vaccine or a placebo, as the research is unknown.” The company told that the participant was reported to be healthy with no adverse occasion after seven days of his dosing, and fitted every criteria in order to be accepted as a volunteer of the trial.

The letter mentioning the death of the 45-year old, pointed out that no follow up was done, and given the moral infringement in the consenting participants, it wasn’t surprising that the volunteers did not feel comfortable to approach the trial site, when they began experiencing symptoms leading to a downfall of their health. The deceased victim’s family has yet not received the individual’s consent form, and after People’s Hospital received the postmortem report, several days later the family got it, stating that the cause of his death was “cardiorespiratory failure due to suspected poisoning.”
On 3rd January, 2021 India gave the green signal and approved the emergency use of the developed vaccines by Bharat Biotech in collaboration with Indian Council of Medical Research and the National Institute of Virology, who manufactured the country’ first indegenious vaccine – Covaxin, and the Serum Institute’s Covishield, developed by the Oxford University and the pharmaceuticals company AstraZeneca.
References:
Picture Sources:
- Scroll
- The Indian Express
- Deccan Herald